Journal of Animal Research: v.10 n.2, p. 269-273. April 2020
DOI: 10.30954/2277-940X.02.2020.15
Isolation of Pseudomonas aeruginosa from Bovine Mastitic Milk Sample Along with Antibiogram Study
ABSTRACT
A total of 4378 cattle and buffalo were screened during period of study (July 2018-June 19). Out of which 27 Pseudomonas aeruginosawere isolated. The isolates were confirmed phenotypically based on pigment production on nutrient agar. These were then confirmed by PCR amplification of species specific oligonucleotide sequences. All the 27 isolates amplified 956bp amplicon 16srRNA Pseudomonas aeruginosaspecies specific nucleotide sequence. The isolates were also checked for exoand aglD virulence associated genes. All of them amplified 540bp and 313bp amplicon of exogene and aglD gene. All the isolates were subjected to antibiotic sensitivity testing. Most of the isolates showed highest sensitivity for levofloxacin, streptomycin and enrofloxacin followed by gentamicin, moxifloxacin and amikacin. Neomycin, cefoperazone and ceftriaxone were intermediate in action.
Keywords: Mastitis, cattle, buffalo, Pseudomonas aeruginosa, virulence genes, antibiogram
Pseudomonas aeruginosais a gram’s negative opportunistic environmental pathogen and can cause severe clinical diseases in both animals and humans. Prevalence of pseudomonas mastitis is only sporadic (<10% prevalence) as reported previously, but it may be a serious herd problem due to persistence of high antibiotic resistance strains (Livermore, 2002; Sharma and Sindhu, 2007). It is typically found in soil, water, skin flora and most man-made environments as it requires minimal amounts of oxygen for growth. Thereby allowing it to colonize a multitude of both natural and artificial environments (Hameed et al., 2007). Contaminated teat dips, teat wipes, and dry-cow intramammary treatment tubes have also been implicated in outbreaks of acute and subacute mastitis in dairy cows, sheep, and goats. Contaminated dry-cow intramammary infusion tubes have been found to be the cause of P. aeruginosamastitis following calving in a dairy herd in Australia with the outbreak resulting in the loss of almost one-third of the herd (death or culling) (Kelly and Wilson, 2016). The presence of unsanitary housing and bedding conditions can contribute to occasional outbreaks of P. aeruginosainfections.
When the host defences are decreased by stress, concomitant disease, or by nutritional imbalances, P. aeruginosaefficiently attacks weak or injured tissues of teats or mammary gland causing acute mastitis with systemic signs as well as subclinical chronic mastitis. In severe cases, the affected gland may be necrotic or even gangrenous and the blood stained milk. Most P. aeruginosastrains possess the type III secretion system (TTSS), which may increase somatic cell counts (SCCs) in milk from mastitis-affected cows. Moreover, most of P. aeruginosabacterial cells can form biofilms, thereby reducing antibiotic efficacy (Park et al., 2014). The pathogen uses a wide range of virulence factors, including proteins capable of inducing toxicity, which effectively damage tissues (Lyczak et al., 2000). Some of these toxic effector proteins are injected into the cytosol of host cells through the type III secretion system (TTSS) which are involved in the disruption of epithelial surface and cell death. These TTSS proteins include cytotoxin (ExoU), ADP-ribosylating enzymes (ExoS and ExoT) and adenylcyclase (ExoY). These cytotoxinsare involved in adhesion, phagocytosis and systemic spread of the bacterial cells together (Vance et al., 2005; Engel and Balachandran, 2009; Hauser, 2009).
How to cite this article: Yadav, R., Chhabra, R., Shrinet, G. and Singh, M. (2020). Isolation of Pseudomonas aeruginosafrom bovine mastitic milk sample along with antibiogram study. J. Anim. Res.,10(2): 269-273.
Outer membrane of P. aeruginosabacterium creates permeability barrier, resultant became naturally resistant to many antibiotics. Moreover, Pseudomonasmaintains antibiotic resistance plasmids, R-factors and resistance transfer fragment (RTFs) and it is able to transfer these genes by horizontal gene transfer (HGT), mainly transduction and conjugation (Livermore, 2002; Kobayashi et al., 2013). This high level of multidrug resistant P. aeruginosastrains are major concern to dairy industries. Although, incidence of P. aeruginosainfection are reported very few (less than 3%) if udder is already infected by Staphylococcusor Streptococcusetc. pathogens, but mastitis remained incurable due to presence of multidrug resistance strains of P. aeruginosa(Ama et al., 2016). On keeping above facts in mind the present study was designed to isolates and identify virulent P. aeruginosafrom mastitic milk of cattle and buffalo along with their antibiogram.
MATERIALS AND METHODS
Sample collection, isolation and identification
Mastitic milk samples of 4378 cattle and buffalo during year 2018-19 were processed for isolation and identification of Pseudomonas aeruginosaisolates. All the samples were plated onto Nutrient agar and incubated at 370C for 24 h. The presumptive isolates were phenotypically characterized on the basis of colony morphology, catalase, oxidase, pigmentation and gram’s staining.
Table 1: Primer sequence for PCR amplification

Molecular confirmation and virulence gene detection of P. aeruginosa isolates
Presumptive Pseudomonas aeruginosaisolates were confirmed by genus and species specific 16S rRNA nucleotide sequence (Spilker et al. 2004). The virulence associated genes were also detected molecularly by exoS and aglD nucleotide sequence as shown in table 1. The reaction mixture (total volume 25μl) was prepared by mixing 12.5μl GoTaq® Green Master Mix (Promega), 1μl Primer-1 and 2 each (10 pM/μl), 3μl Template DNA (25ng/ μl) and 7.5μl Nuclease free water (upto final volume 25μl). PCR Amplification was carried out in ‘Thermal cycler gradient (BR Gradient Thermal Cycler)’ as follows: initial cycle of denaturation at 95 °C for 5min, 30 cycle at (denaturation at 95 °C for 45 s, primer annealing at 55 °C for 45 s and primer extension at 72 °C for 60 s), and final extension at 72 °C for 5 min. The PCR products were analyzed by electrophoresis on 1-1.5% agarose gel with ethidium bromide (0.5 μg/ml) in 1X TAE buffer for 60 min at 100 V. The gel was then visualized by Azure C-15 UVP gel documentation system.
Determination of antibiotic resistant strains of P. aeruginosa isolates
The antibiotic sensitivity testing was carried out as per standard protocol describe by Kirby et al. (1966) against 16 different antibiotics. Briefly, the isolates were inoculated in sterile BHI broth (5 ml) and incubated for 18-24 h at 37°C. The opacity of 0.5 McFarland opacity standards (Quinn et al., 2002) was well spread over the Muller Hinton agar with the help of sterilized swab and after that antibiotic discs were carefully placed, then incubated for 24 h at 37°C. The zone of inhibition was measured for each disc and interpretation was done as per Clinical and Laboratory Standards Institute (CLSI) guidelines (Wayne, 2009). All Multidrug resistant (MDR) isolates were evaluated for their multiple antibiotic resistances (MAR) index (Krumperman, 1983).
RESULTS AND DISCUSSION
Pseudomonas aeruginosais becoming a threat to dairy animals causing mastitis by drug resistance strain, since it requires few nutrients to multiply. It is the most commonly isolated microorganisms from clinical specimens, usually responsible for nosocomial infections (Chattergee et al., 2016). In the present study, 27 (17 from buffalo and 10 from cattle origin) Pseudomonas aeruginosawere isolated from mastitic milk samples of 4378 cattle and buffalo during year 2018-19. All the isolates were catalase and oxidase positive and produce green pigmentation on nutrient agar plate. Further, molecular confirmation was done by PCR amplification of genus and species specific oligonucleotide sequences (Fig. 1). All 27 isolates amplified 618bp amplicon and 956 bp amplicon of 16S rRNA Pseudomonasgenus specific and 16S rRNA P. aeruginosaspecies specific nucleotide sequence. The prevalence of P. aeruginosawas detected as 0.61% in the present study. In agreement to our study, Nam et al. (2009) reported 5.6% prevalence of P. aeruginosafrom bovine mastitis between 2003 and 2008 in Korea and Vasquez-Garcia et al. (2017) detected 9.6% prevalence of P. aeruginosafrom bovine in Brazil. Banerjee et al. (2017) also detected 5.4% cases of bovine subclinical mastitis in South Bengal, India were associated with P. aeruginosa. Contrarily, Scaccabarozzi et al. (2015) detected high prevalence (nearly 27%) of P. aeruginosaas causative agent of mastitis in goats.
All the 27 P. aeruginosaisolates amplified 313bp amplicon of aglD virulence associated genes and 540bp amplicon of exoenzyme, exoS gene which helps in invasion. exoS and exoT are two homologous bifunctional Type III Secretion System (T3SS) virulence factors that induce apoptosis in target host cells (Kaminski et al. 2018).
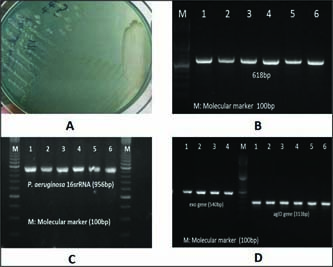
Fig. 1: Growth of P. aeruginosaon nutrient agar plate, PCR amplification of 16SrRNA genus specific (B), 16SrRNA P. aeruginosaspecific specific (C) and virulence genes exoSand aglD gene (D)sequences
Similar to our observation Park et al. (2014) also detected TTSS-related genes (exoU and/or exoS genes) in 82.7% of the isolates including the invasive (exoU-/exoS+, 69.4%), cytotoxic (exoU+/exoS-, 8.3%) and cytotoxic/ invasive strains (exoU+/ exoS+, 5.0%). The aglD gene of P. aeruginosais responsible for synthesis of the capsular polysaccharide alginate, an important virulence factor expressed in cystic fibrosis (CF) cases (Baynham et al. 1996). Similar to present study, Taee et al. (2014) 98% detection of aglD gene by PCR amplification from P. aeruginosaof clinical isolates.
All the isolates were tested against 16 antibiotics for antibiotic sensitivity. All the isolates showed absolute resistant to chloramphenicol, penicillin-G, cloxacillin, tetracycline, amoxycillin, kanamycin and ampicillin. The isolates were most sensitive to levofloxacin, streptomycin, enrofloxacin followed by gentamicin, moxifloxacin and amikacin (Fig. 2). Neomycin, cefoperazone and ceftriaxone were intermediate in action. Standard antibiotic regimes against P. aeruginosaare increasingly becoming ineffective due to the rise in drug resistance (Chattergee et al., 2016). Park et al. (2014) detected efficiency of antibiotic therapy against P. aeruginosarelated bovine mastitis and found that majority of isolates were sensitive to gentamicin, amikacin, meropenem and ciprofloxacin. They also suggested that determination of efficacy could also be improved by MIC analysis of the isolates.
In contrast to our observation, Smith et al. (2012) reported that all the 20 P. aeruginosaisolates from cystic fibrosis cases (humans) showed resistance to gentamicin (80%) and ciprofloxacin (70%). However, detection of resistance towards amoxycillin (100%), tetracycline (95%), augmentin (95%), ofloxacin (80%), nalidixic Acid (100%), was similar to observations in the present study. Imipenem was the drug of choice for treatment of cystic fibrosis previously but increasing trends in imipenem resistance leaves fluoroquinolones only in terms of susceptibility. The cephalosporins tested were showing emergence of resistance due to indiscriminate use.
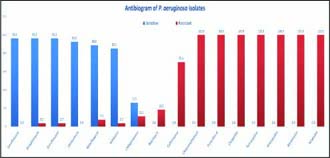
Fig. 2: Graphical representation of antibiotic sensitivity and resistance patterns of P. aeruginosaisolates
CONCLUSION
From this study, majority of isolates were highly pathogenic on the basis of detection of exoS and aglD virulence genes and also resistant to majority of antibiotics (eight antibiotics) signifying constant antibiotic screening must be done before mastitis treatment for Pseudomonas aeruginosainfections.
CONFLICT OF INTEREST
Authors don’t have any conflict of interests.
REFERENCES
Ama, A., El-Shafi, S.S.A., Elwahab A.M.O. and El-dayim, Z.A.A. 2016. Be detection of multidrug resistance genes in Pseudomonas aeruginosaisolated from bovine mastitic milk. J. Dairy. Vet. Anim. Res.,3(2): 43‒49.
Banerjee, S., Batabyal, K., Joardar, S.N., Isore, D.P., Dey, S., Samanta, I., Samanta, T.K. and Murmu, S. 2017. Detection and characterization of pathogenic Pseudomonas aeruginosafrom bovine subclinical mastitis in West Bengal, India. Vet. World.,10(7): 738.
Baynham, P.J. and Wozniak, D.J. 1996. Identification and characterization of AlgZ, an AlgT-dependent DNA-binding protein required for Pseudomonas aeruginosaalgD transcription. Mol. Microbiol.,22(1): 97-108.
Bogiel, T., Deptula, A., Kwiecinska-pirog, J.O.A.N.N.A., Prażynska, M., Mikucka, A. and Gospodarek-Komkowska,
E. 2017. The prevalence of exoenzyme S gene in multidrug- sensitive and multidrug-resistant Pseudomonas aeruginosaclinical strains. Polish J. Microbial.,66(4): 427-431.
Chatterjee, M., Anju, C.P., Biswas, L., Kumar, V.A., Mohan, C.G. and Biswas, R. 2016. Antibiotic resistance in Pseudomonas aeruginosaand alternative therapeutic options. Int. J. Med. Microbiol.,306(1): 48-58.
Engel, J. and Balachandran, P. 2009. Role of Pseudomonas aeruginosatype III effectors in disease. Curr. Opin. Microbiol.,12: 61–66.
Hameed, K.G.A., Sender, G. and Korwin-Kossakowska, A. 2007. Public health hazard due to mastitis in dairy cows. Ani. Sci. Paper. Reports.,25(2): 73-85.
Hauser, A.R. 2009. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nature. Rev. Microbiol.,7(9): 654.
Kaminski, A., Gupta, K.H., Goldufsky, J.W., Lee, H.W., Gupta, V. and Shafikhani, S.H. 2018. Pseudomonas aeruginosa ExoSinduces intrinsic apoptosis in target host cells in a manner that is dependent on its GAP domain activity. Sci. reports.,8(1): 14047.
Kelly, E.J. and Wilson, D.J. 2016. Pseudomonas aeruginosamastitis in two goats associated with an essential oil–based teat dip. J. Vet. Diag. Invest.,28(6):760-762.
Kirby, W.M., Bauer, A.W., Sherris, J.C. and Turck, M. 1966. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol.,45(4): 493-496.
Kobayashi, H., Isozaki, M., Fukuda, T., Anzai, Y. and Kato,
F. 2013. Surveillance of fluoroquinolone-resistant clinical isolates of Pseudomonas aeruginosa. Open J. Med.Microbiol.,3(02): 144.
Krumperman, P.H. 1983. Multiple antibiotic resistance indexing of Escherichia colito identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol.,46(1): 165-170.
Livermore, D.M. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis.,34(5): 634-40.
Lyczak, J.B., Cannon, C.L. and Pier, G.B. 2000. Establishment of Pseudomonas aeruginosainfection: lessons from a versatile opportunist. Microbes. Infect.,2: 1051–1060.
Nam, H.M., Lim, S.K., Kang, H.M., Kim, J.M., Moon, J.S., Jang, K.C., Joo, Y.S. and Jung, S.C. 2009. Prevalence and antimicrobial susceptibility of gram-negative bacteria isolated from bovine mastitis between 2003 and 2008 in Korea. J. dairy. Sci.,92(5): 2020-2026.
Park, H.R., Hong, M.K., Hwang, S.Y., Park, Y.K., Kwon, K.H., Yoon, J.W., Shin, S., Kim, J.H. and Park, Y.H. 2014. Characterisation of Pseudomonas aeruginosarelated to bovine mastitis. Acta. Vet. Hung.,62(1): 1-12.
Quinn, P.J., Markey, B.K. and Carter, M.E. 2002. Veterinary microbiology and microbial disease. Ames, Iowa: Iowa State University Press.
Saadoon, Z.S. and Zghair, Z.R. 2019. Molecular Detection of Pseudomonas aeruginosaby Using Algd, Plch and Lasb Genes and Pathological Study of the Virulent Isolate from Human Blood. Plant. Archives.,19(2): 1633-1639.
Scaccabarozzi, L., Leoni, L., Ballarini, A., Barberio, A., Locatelli, C., Casula, A., Bronzo, V. , Pisoni, G., Jousson, O., Morandi, S. and Rapetti, L. 2015. Pseudomonas aeruginosain dairy goats: genotypic and phenotypic comparison of intramammary and environmental isolates. PloS one., 10(11): p.e0142973.
Sharma, A. and Sindhu, N. 2007. Occurrence of clinical and subclinical mastitis in buffaloes in the State of Haryana (India). Ital. J. Ani. Sci.,6: 965-967.
Smith, S., Ganiyu, O., John, R., Fowora, M., Akinsinde, K. and Odeigah, P. 2012. Antimicrobial Resistance and Molecular Typing of Pseudomonas aeruginosaIsolated from Surgical Wounds in Lagos, Nigeria. Acta. Med. Iran.,50(6): 433-438.
Spilker, T., Coenye, T., Vandamme, P. and LiPuma, J.J. 2004. PCR-based assay for differentiation of Pseudomonas aeruginosafrom other Pseudomonas species recovered from cystic fibrosis patients. J. Clin. Microbiol.,42(5): 2074-2079.
Taee, S.R., Khansarinejad, B., Abtahi, H., Najafimosleh, M. and Ghaznavi-Rad, E. 2014. Detection of algD, oprLand exoAgenes by new specific primers as an efficient, rapid and accurate procedure for direct diagnosis of Pseudomonas aeruginosastrains in clinical samples. Jundishapur. J. Microbiol.,7(10): e13583.
Vance, R.E., Rietsch, A. and Mekalanos, J.J. 2005. Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosaPAO1 in vivo. Infect. Immun.,73: 1706–1713.
Vasquez-Garcia, A., Silva, T.D.S., Almeida-Queiroz, S.R.D., Godoy, S.H., Fernandes, A.M., Sousa, R.L. and Franzolin, R. 2017. Species identification and antimicrobial susceptibility profile of bacteria causing subclinical mastitis in buffalo. Pes. Vet. Brasil.,37(5): 447-452.
Wayne, P. 2009. Clinical and Laboratory Standards Institute (CLSI) performance standards for antimicrobial disk diffusion susceptibility tests 19th ed. approved standard. CLSI document M100-S19, 29 (2011), M100-S21.